A galvanic cell, or voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from . This arrangement is called a galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed .

A Voltaic Cell (also known as a Galvanic Cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A summary of Galvanic Cells in ‘s Galvanic Cells. Learn exactly what happened in this chapter, scene, or section of Galvanic Cells and what it means.
Electrochemistry and electrochemical cells were introduced in our unit on diabetes monitoring.

All about Galvanic Cells, which are also called Voltaic Cells. These are devices that use a chemical reaction. Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells, and common batteries consist of one or more such cells. Electrochemistry: Galvanic Cells and the Nernst Equation.
In the movie on the previous page, when a zinc . Explain chemical reactions for each electrode of a battery or galvanic cell. Describe oxidation and reduction reactions. Learn how different types of electrochemical cells work.
Diagrams and explanations of galvanic and electrolytic cells are provided.
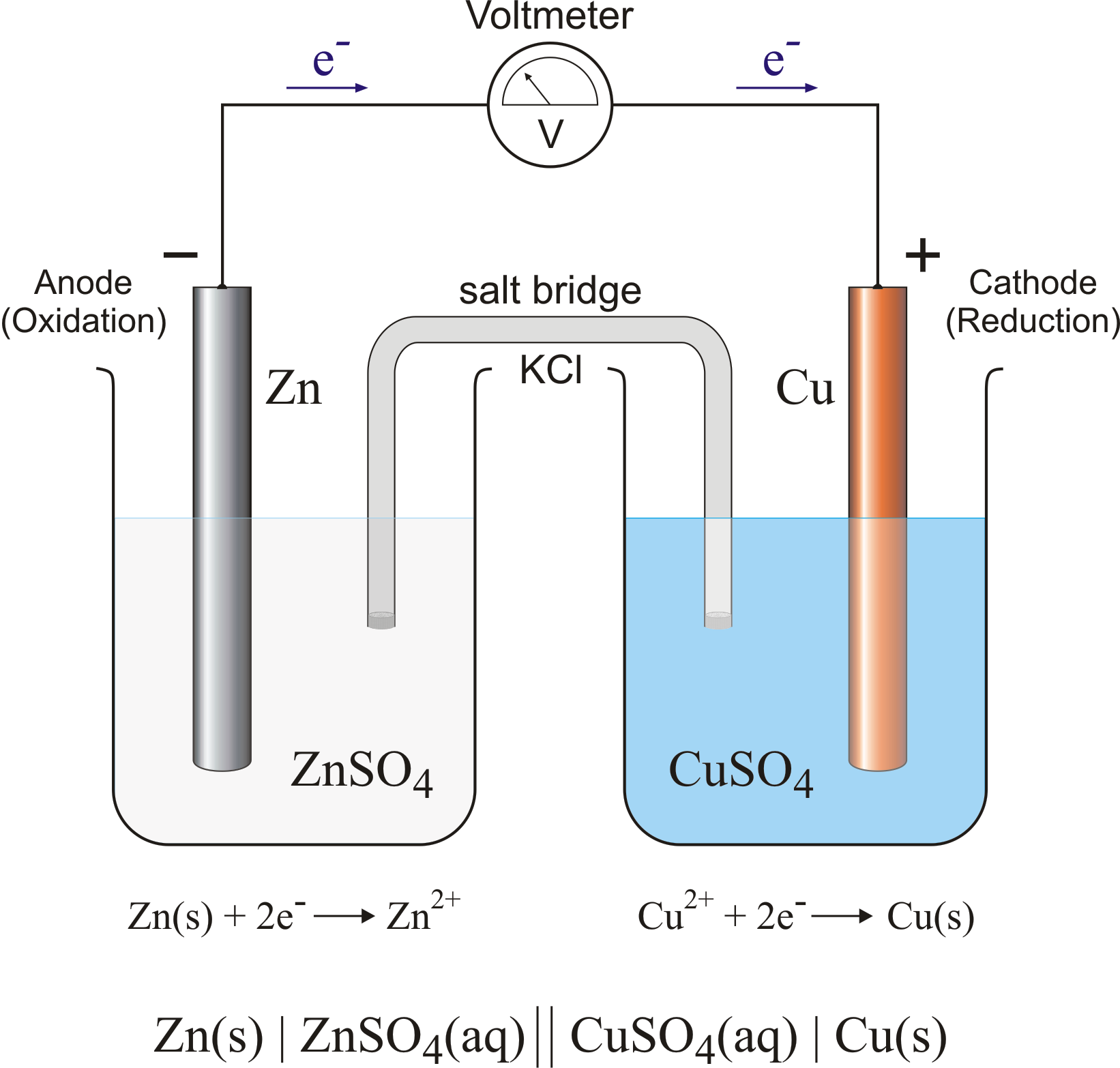
In the previous step, we harnessed the energy of . In the preceding simulations you measured cell . The term galvanic cell does not exist in the database. Displaying of the search for galvanic+cell. The database contains chosen terms and concepts, . Galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy. Goal: to describe the construction and operation of a voltaic cell.
Learn more about voltaic cells in the Boundless open textbook. A voltaic cell is a device that produces an electric current from energy released by a spontaneous . A galvanic cell is a spontaneous electrochemical cell that produces electricity by a spontaneous redox reaction. To complete the circuit, a salt bridge is require .


